
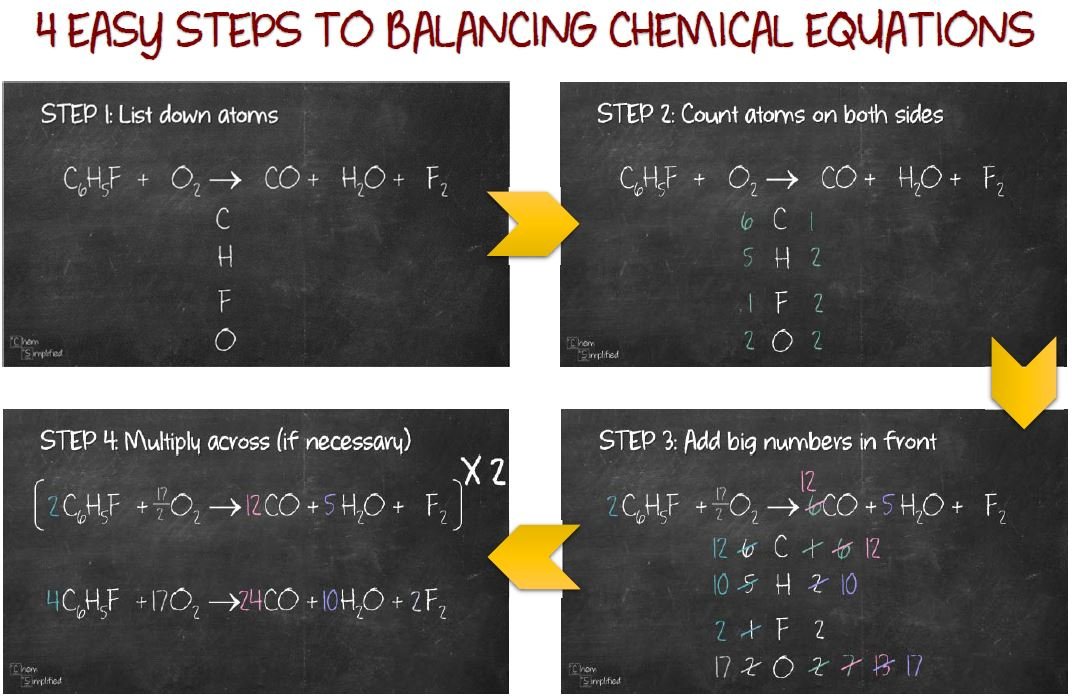
This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. Balancing EquationsĪ balanced chemical is equation has equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. Regardless of the absolute number of molecules involved, the ratios between numbers of molecules are the same as that given in the chemical equation. One mole of methane molecules and 2 moles of oxygen molecules react to yield 1 mole of carbon dioxide molecules and 2 moles of water molecules.įigure 2.One dozen methane molecules and two dozen oxygen molecules react to yield one dozen carbon dioxide molecules and two dozen water molecules.One methane molecule and two oxygen molecules react to yield one carbon dioxide molecule and two water molecules.Likewise, these coefficients may be interpreted with regard to any amount (number) unit, and so this equation may be correctly read in many ways, including: This ratio is satisfied if the numbers of these molecules are, respectively, 1-2-1-2, or 2-4-2-4, or 3-6-3-6, and so on (Figure 2). Methane and oxygen react to yield carbon dioxide and water in a 1:2:1:2 ratio. Realize, however, that these coefficients represent the relative numbers of reactants and products, and, therefore, they may be correctly interpreted as ratios. It is common practice to use the smallest possible whole-number coefficients in a chemical equation, as is done in this example. The relative numbers of reactant and product species are represented by coefficients (numbers placed immediately to the left of each formula).
Chemical equation balancer calc plus#

The preceding chapter introduced the use of element symbols to represent individual atoms.
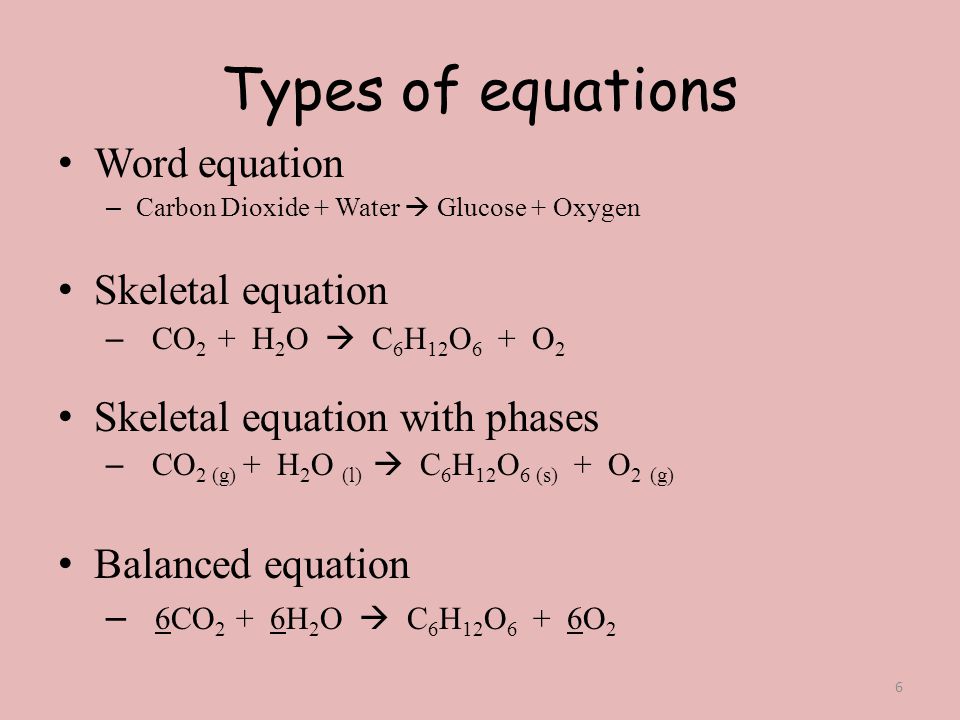
Write and balance chemical equations in molecular, total ionic, and net ionic formats.Derive chemical equations from narrative descriptions of chemical reactions.Please let us know how we can improve this web app.By the end of this section, you will be able to: calcium hydroxide + carbon dioxide = calcium carbonate + waterĮxamples of the chemical equations reagents (a complete equation will be suggested):.PhCH 3 + KMnO 4 + H 2SO 4 = PhCOOH + K 2SO 4 + MnSO 4 + H 2O.To enter an electron into a chemical equation use + H 2O.Compare: Co - cobalt and CO - carbon monoxide Always use the upper case for the first character in the element name and the lower case for the second character.Ğxamples: Fe, Au, Co, Br, C, O, N, F.Enter an equation of a chemical reaction and click 'Balance'.Instructions on balancing chemical equations:


 0 kommentar(er)
0 kommentar(er)
